- Home
- About Us
- Service
- Application
- Technology
- Resource
- Contact Us

HapOnco® mClear
HapOnco® mClear
Personalized MRD Testing for Solid Tumors
Product Introduction
HapOnco® mClear Personalized MRD Testing for Solid Tumors is a MRD gene testing product independently developed by HaploX. Based on the detection results of HaploX's star product WESPlus® and the comprehensive design of patient-specific gene panels with important tumor hotspot mutations, it uses multiple core technologies such as ultra-high-depth sequencing, multiple noise reduction strategies, and the independently developed MRD bioinformatics algorithm VarSingal™ to help accurately detect ultra-low-frequency MRD, and to timely and accurately monitor the changes in the level of MRD in patients, providing clinicians with more comprehensive and reliable information for clinical treatment decision-making.

Product Content
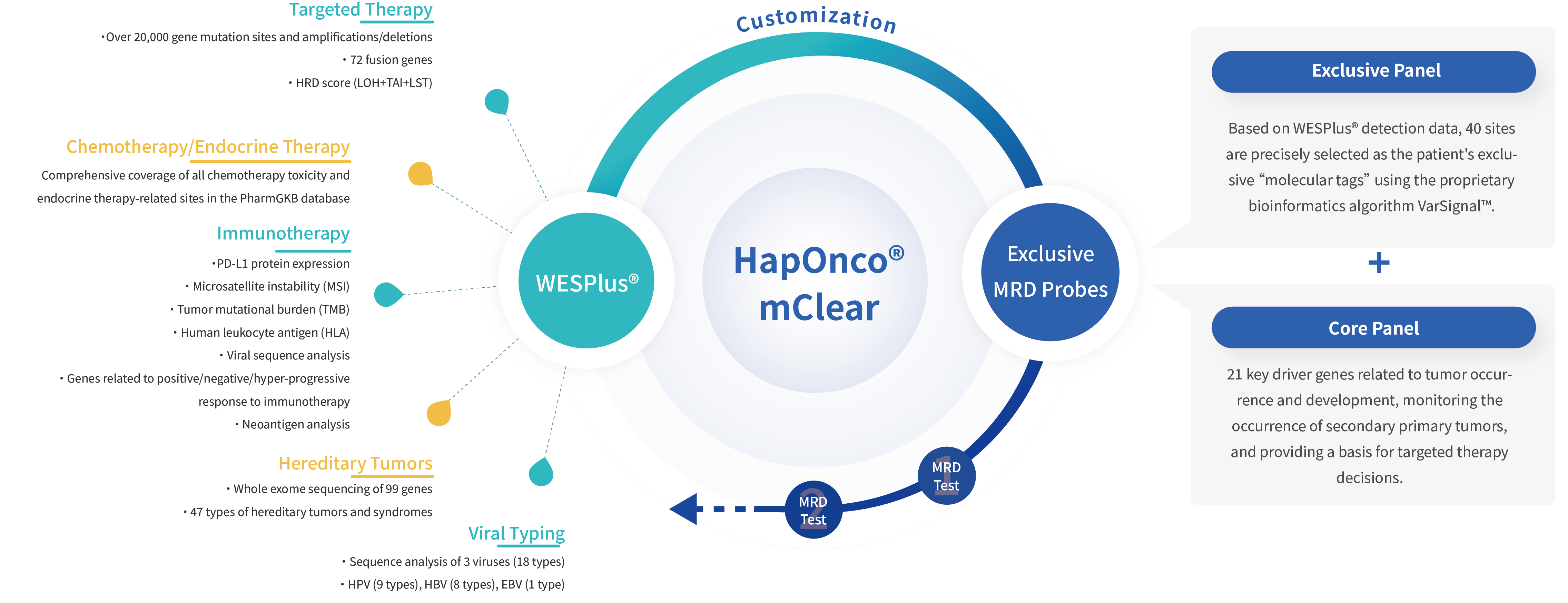
HapOnco® mClear first uses the whole - exome sequencing gene testing product WESPlus® to detect the tumor tissue samples of patients and comprehensively obtain gene mutation information. It accurately selects up to 40 mutation sites as the patient's exclusive "molecular tag" and customizes exclusive MRD probes in combination with important tumor driver genes. It uses differentiated dual - gene panel design to evaluate MRD, which combines the two MRD detection technologies of informed analysis based on tumor - acquired information (that is, the informed analysis through patient tumor sequencing) and uninformed analysis not affected by tumor information (that is, the non - informed analysis based on the genetic composition of the patient's tumor). It takes advantage of both to ensure the stability and reliability of HapOnco® mClear MRD detection. The customized probes are used to dynamically monitor MRD in the patient's blood samples at an ultra - high depth of 100,000X, accurately identify ultra - low - frequency mutations, monitor the recurrence of tumors and the occurrence of secondary primary tumors, provide early tumor recurrence warning ahead of radiological indications for patients, provide a basis for clinical treatment decision - making, and grasp the best treatment timing.
Product enables
![]() Assessment of treatment and drug regimes
Assessment of treatment and drug regimes
![]() Prognostic risk stratification for more accurate and personalized treatment plans
Prognostic risk stratification for more accurate and personalized treatment plans
![]() Efficacy assessment of postoperative & adjuvant therapy
Efficacy assessment of postoperative & adjuvant therapy
![]() Drug resistance monitoring to suggest drug resistance mutation generation
Drug resistance monitoring to suggest drug resistance mutation generation
![]() Dynamic recurrence monitoring to detect progression earlier than imaging
Dynamic recurrence monitoring to detect progression earlier than imaging
![]() Indication of potential entry into drug holidays based on MRD test results
Indication of potential entry into drug holidays based on MRD test results
Product Advantages
(1)Non-invasive, dynamic, and sensitive MRD detection:Compared with other main methods of MRD assessment, ctDNA-NGS-based MRD detection is a non-invasive, dynamic, and more sensitive method, which can track cancer progression during treatment, assist in the selection of treatment strategies, and longitudinally monitor the risk of cancer recurrence when patients achieve remission.
(2)Ultra-high depth: HapOnco® mClear MRD testing achieves an ultra-high sequencing depth of 100000X, and has conducted rigorous multiple repeat experiments on standards to verify that the minimum detection limit of HapOnco® mClear reaches 0.005%.
(3)Reliable data: Thanks to the strong experimental and bioinformatics teams, HapOnco® mClear integrates multiple technologies to reduce background noise at each step of the entire process, ensuring reliable data results.
Applicable Population
![]() Patients with early - to - mid - stage resectable solid tumors who need long - term monitoring of tumor progression
Patients with early - to - mid - stage resectable solid tumors who need long - term monitoring of tumor progression
